Myelodysplastic syndromes / MDS
Myelodysplastic syndromes is a term we use for a wide group of malignant diseases of the hematopoietic tissue that can differ from each other but all have some main common characteristics : All of the myelodysplastic syndromes are diseases of the hematopoietic tissue and they are caused by a clonic disorder of the multipotent stem cell. Normally, the stem cells when they differentiate they “give” one stable line of undifferentiated cells and a line of cells that will eventually evolve into a type of blood cells. Multipotent stem cells have a very high capacity of self -renewal and they have the ability to differentiate into different cell types in an adult. So basically, the first adult hematopoietic cells can start differentiating out of the normal line, causing different types of syndromes.
Let’s take a look now, in some other common features
these syndrome have :
There is ineffective hematopoiesis, we see Cytopenia so there aren’t being produced enough cells for some blood types, the bone marrow is full of cells, the Cells have various morphological disorders, we have different percentage of blasts in the bone morrow in comparison with the percentage of a normal person , we see chromosomal abnormalities that have not been inherited and lastly there are some myelodysplastic cases that will eventually progress in becoming acute melogenic leukemia. (Connection between Myelodysplastic syndromes and AML). It is considered that as the person grows older, so does the risk of developing the disease grows. Further, myelodysplastic syndromes can be developed as a primary disease, but they can also appear as secondary as a result of some medications.
How
does a MDS develop?
One stem cell,
a multipotent hematopoietic stem cell, ends up with damaged genetic material
that is irreversible. This damage that is
often found lies in transcription factors, in histones or in DNA methylation.
There is also the Methylation of P15
(INK4B) , a tumor suppressor gene that is found in 30-50% of cases of MDS and
seems to be related to the DNA methylation . In essence it is an epigenetic glitch, the sequence
of genes does not change but the expression gets altered.
These damages make the cell more powerful and
advantageous over the other normal stem cells.
As it starts multiplying and generating more copies of itself, the bone marrow
eventually becomes flooded with this particular cell and its daughter cells. In
addition, our mutated cell can change the expression of MHC molecules on its
surface so that it can avoid an immune response, getting away from our immune
system.
Further, this
“problematic” cell will cause a lot of changes to our cell mechanisms. For
example, the metabolic pathways of stimulus treatment get altered so they will not
be affected by apoptotic factors, while the normal cells are quite sensitive and
responsive to them. The result is, this cell and its progeny will prevail over
other normal cells. Equally important is to note that, the cancerous stem cells
create changes to growth factors and to the RAS molecule, which is an intracellular
signal molecule that provokes cell proliferation. Basically, mutations in this
molecule promote carcinogenesis.
The
MDS PARADOX
The bone
marrow of a person with MDS is filled with blasts and stem cells and cells in
general with cytopenia in peripheral blood while at the same time there is an
increased proliferation and apoptosis in hematopoietic cells.
Patients who
show a High rate of stem cell apoptosis often have "good" prognosis of the syndrome. Basically,
it is very important for the cells to not lose their ability of apoptosis.
Apoptosis is the
programmed cell death. In order for a cell to do apoptosis, it needs to consume
energy and develop morphological characteristics
such as chromatin contraction, oligomeric changes in DNA, nuclear envelope
fragmentation and cellular condensation. Basically the cell is cut down into
much smaller sections and these sections will later be phagocytosed. It is a
way of "silent suicide" without disturbing the cell environment.
Apoptosis is
very important of a healthy organism because it keeps the number of tissue
cells at the right level. All cells must have the ability to go through apoptosis.
It is crucial not the confuse apoptosis with cell necrosis, which is a cell destruction
which isn’t silent and will disturb the surrounding environment.
So, MDS cells
do have a high ability to apoptosise. There an increasement of a tumor necrosis
factor , TnF-α
, which is basically a cytotoxic factor that causes cancer cell to
terminate and has been found to rise in MDS/
Additional,
there is an Increased expression of the Fas and Fas-Ligand (FasL) gene on
CD34+. Those are , signals that trigger apoptosis.
Also, there
has been found an augmentation of c-myc/bcl-2 or bax/bad versus bcl-2/bcl-x ,
basically a rise in the expression of apoptotic genes in comparison to anti apoptotic genes.
So in the
end, what can be seen, is that those syndromes have the benefit of a high level
of apoptosis which can lead to a good prognosis of the disease. The paradox
however remains, due to fact that we still see proliferation and apoptosis.
The reason
why hematopoiesis is not properly taking place is not fully understood, since there
is dilemma emerging. It is not clear, if the problem arises exclusively because
of the mutated - problematic but more aggressive and stronger stem cells or if
the T lymphocytes unknowingly help the whole process. There has been found that
T lymphocytes can suppress BFU-E colonies which are precursors of red and
granulocyte cell line colonies as well as CD8+ lymphocytes are able to suspend
the evolution of CFU-GM colonies. Another essential element in the whole case, seems
to be that patients who were given immunosuppressive drugs have shown a better
response than other patients who got different kind of treatments.
But how do
lymphocytes help cancer cells?
One mechanism that has been suggested is that
T-lymphocytes participate in the process and in the development of MDS. In detail, they help the mutated clone
to avoid the immune system.
The T-cells
are the first ones to recognize the cancerous stem cells through MHC molecules (molecules
of our own cells that recognize whether a cell is self-contained or foreign).
Since the T cells recognize the problematic cell they
start to proliferate and to secrete cytokines in order to suppress
hematopoiesis, in order to this specific mutated cell to not be cloned.
But at the same time they help the cancer cell
to secrete, produce and express proteins which will prevent the immune system cells to
realize which is the cancer cell and as a result the mutated stem cell will be
able to “hide” from the immune response. This phenomenon does not seem so
strange anymore because it has been found that general cancer cells have the ability to use elements of the body
against itself as well as using molecules of the body's defense against normal
cells.
Cancer cells
grow in the bone marrow in a specific microenvironment which includes:
Cytokines
A number of cytokines, IL-6, IL-8, SCF, EPO,
TGF-β, GM-CSF, TNF-α, were measured in
the serum and marrow of patients with MDS , with unclear and often conflicting
results.
There is an
increased secretion TNF-α by macrophages and T lymphocytes in the marrow. This
secretion was associated with a high expression of the Fas antigen, a pathway that
also leads to apoptosis. Basically the FAS binds to FAS-L which will active some
proteins called prokaspases and caspases which are proteases that lead to cell
death , by CD34 + cells.
In short, in the environment of bone marrow T lymphocytes produce cytokines cytokines that will prevent hematopoiesis by enabling the apoptosis. In the same time, those cytokines are perceived by the cancer cells, which will produce other molecules that will make them invisible to the immune system. And that’s the only explanation there is for one part of this paradox phenomenon : This type of cancer appears with high abundance of apoptosis which can be a good thing but also this the reason why the cancer cells are able to get away from the immune system. Due to this fact, it is very difficult for a cure to be found, since the scientists can not predict how the disease will evolve while the cancer cells try to “fool” our system.
Angiogenesis
is a complex process that takes part in the formation and creation of cancer.
The density of new blood vessels is higher in MDS in comparison with other
types of Leukemia. In fact, the aggressiveness of a cancer types goes hand with
more intense angiogenesis. This is not a surprise, since the main role of cancer
cells is to find a way to get into the blood circulation. As it is expected,
high levels of VEGF appear, which is a vascular endothelial growth factor that
helps in angiogenesis and increases the vascular permeability. Therefore, the enlargement
of angiogenesis and VEGF is often a sign of a bad prediction of the disease.
Next,
I am going to present some possible reason that are considered to be the causes
of those syndromes by the research community:
As mentioned
above, myelodysplastic syndromes can appear due to borht primary and secondary
causes. However, the majority of these diseases are primary, they appear on
their own, without being the result of a drug that has already been given to
the person.
Primary
Myelodysplastic syndromes can be caused because of:
Heredity
Familial
monosomy 7 is the lack of the long arm of chromosome 7
Trisomy
8 mosaicism: This is disorder
in the mitotic separation of
chromosomes. Depending on when this disorder occurs either in the first
division, the cell appears to be mosomal or in the second where we have
trisomy. The person can also have half
normal karyotype, ¼ trisomy, ¼ monosomy.
Kostmann,
Schwachman-Diamond
Fanconi
anemia, Bloom syndrome
Neurofibromatosis
1
Embryonal
dysgenesis (del12p)
Moreover, cytogenetic lesions can be found, with 70% of them
occurring in primary MDSL while 90% in secondary MDS. Those abnormalities mainly
have to do with chromosome segment
losses .
The Karyotype
can show some cytogenetic lesions. Applying fluorescent in situ is a way in
which we can mark certain areas to see if there are permutations between
chromosomes, changes between the genetic material of chromosomes.
Smoking
Myelodysplastic syndromes relate with the duration of
smoking. Consequently, a person is actually in high risk of developing MDS 15
years even after they stop smoking.
Secondary causes of myelodysplastic syndromes:
Ionizing radiation
Patients who
have been radiated in the pelvis and spine, people who have been near atomic
explosions, or even in near countries, patients who have received radioactive
phosphorus. It is important to be noted that someone can develop MDS 17 years
after the radiation.
Benzene or benzene
The exposure
to gasoline and other petroleum products from 1-15 years can increase the risk
of developing MDS.
chemotherapeutic
drugs
The
alkylating agents chlorambucil, cyclophosphamide, melphalan, nitrosures,
busulfan, procarbazine can be administered to a patient and it is considered
that he may develop a myelodysplastic syndrome 5 to 7 years after the
chemotherapy with the maximum risk between the 2ed and the 5th year.
In essence, these agents have a cytotoxic effect. Their action is related to
their ability to form strong covalent bonds with DNA bases and interfere with
the normal functioning of DNA. Τhey can act non-specifically and create "cross bridges"
so that the process of replication or transcription can not be done properly. They
can also act specifically which in some cases results in mutations while the
cell is trying to correct these changes through repair enzymes often ends up
creating DNA fragments because it can
not deal with the mutations or mistakenly bind the bases. So ultimately, the
way Alkylating agents act is either by blocking transcription and replication,
or by acting directly on mutations, or by leading to DNA fragments
It is
important to be noted that those agents, are often prescribed to cancer
patients in cancer treatment programs and their main goal is to keep the cell
from reproducing since it damages its DNA. So basically, those drugs are
essential for a cancer patient and they are important to the survival rate. However,
in some cases, they can contribute to further damage of normal stem cells and
end up mutating them, creating a new cancer disease for the patient. A vicious circle
which is created and harnessed by cancer.
Comparing Smoking – benzene-chemotherapy drugs:
According to recent
studies there are evidence showing that MDS increases more among smokers due to
the presence of polycyclic hydrocarbons (benzene) and many other carcinogens in
tobacco. Also, people who develop MDS after being affected by benzene, have a similar
disease growth to those who develop the syndrome after chemotherapy with
alkylating agents. The reason why is that petroleum products have a similar
molecular acting as the alkylating agents have. In the end, smoking can be
compared with inhaling benzene and taking a heavy chemotherapeutic drug.
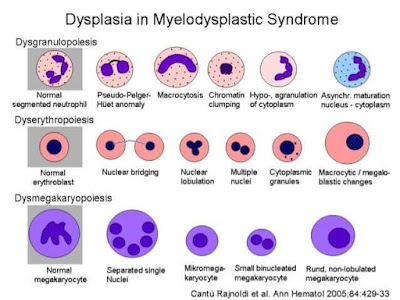
Cell
morphology in MDS
Τhere
is a disruption in all cell lines.
Dyserythropoiesis
In peripheral
blood, we can see dimorphism, double population of red blood cells,
Anisocytosis – multicellularity which means many types of morphological changes
in erythrocytes. Ovarian macrocytes, elliptocytes, tear cells, erythroblasts
with megaloblastic characteristics, like a change in the proportion of nucleus to
cytoplasm, insufficient hemoglobinization of erythrocytes. All of these indicate a disorder in the maturity
of red blood cells, with genetic material residues.
In the bone
marrow we can see erythroblasts nucleus with more lobes, or karyorixia, where the
shrunken nucleus breaks down and disappears completely. In addition, there is a
change in the ratio of nucleus to the cytoplasm, there are Ring iron blasts
where rings appear around the nucleus. This is caused by a disturbance in the
intake of iron, the cell takes up more iron in the mitochondria and as a result,
the mitochondria go around the nucleus and create this ring.
Disgranulopoiesis
In Peripheral
blood we can see neutropenia, reduced cytoplasm granulation , myeloperoxidase negative, a reduction In the
nucleus lobes with an increased density and
thickening of the chromatic (pseudo-Pelger-Huet abnormality). An over segmentation
of the nucleus is seen, as well as basophilia in the perimeter of the
cytoplasm. In some cases there is an increased granulation of the cytoplasm with
large azurophilic granules instead of the usual ones (pseudo Chediac Higashi).
Lastly, we can see blasts with or without Auer sticks. (Note that blasts shouldn’t
be seen in the blood)
In the bone
marrow, we can see hyperplasia, with the premature forms of the granular cell
line being dominant while suffering from maturity both from the nucleus and the
cytoplasm.
Dismegakaryopoiesis
In the Peripheral
blood we see thrombocytopenia, Hypocranial or non-granular platelets ( basically
platelets with little or absent granules), Giant platelets, Pathological functioning platelets. Also, megakaryocyte
fragments will appear. Note that, megakaryocytes do not normally exist in the
peripheral blood , only in the bone marrow and from them , the platelets will
be created , and these are the ones we are supposed to see in the blood.
In the bone Marrow
we can see micromegakaryocytes (<20μm). Normally they are quite large while
in the MDS they will appear quite small. They will have multiple small nuclei and with less granules .
A mononuclear form with a small round eccentric nucleus with a cell diameter of
less than 30 μm is associated with the cytogenetic abnormality 5q.
0 comments