Hematopoiesis (Part one)
Hematopoiesis is the process where the mature blood
cells of our body are produced. This procedure begins in the embryonic stage
and every cell develops from the hematopoietic stem cells (HSCs). It should be
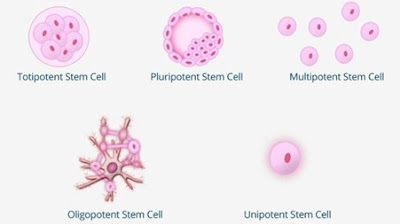
noted that there are five different types of stem cells:
Totipotent (or Omnipotent) Stem Cells:
They are the most powerful ones since they have the ability to differentiate
into embryonic and extra embryonic types of cells like the placenta. In other
words, they can produce each cell in our bodies and form a whole new organism.
Pluripotent Stem Cells:
These cells are considered the "offspring" of totipotent stem cells.
They can also develop into any kind of cell in our body, but they cannot form
extra outer embryonic tissues.
Multipotent Stem Cells:
They have the ability to differentiate into relative cells of the same tissue,
like for instance the erythrocytes, leukocytes, platelets etc.
Oligopotent Stem Cells: These stem cells can differentiate into cell
types of the same cell line. Such examples are the myeloid stem cells.
Unipotent Stem Cells: They can
only produce one type of cell, since they have the lowest differentiative potential. For instance, they can only produce sperm
cells.
https://bioinformant.com/do-you-know-the-5-types-of-stem-cells-by-differentiation-potential/
The first steps of hematopoiesis begin as early as the
7th day of the embryonic life with the appearance of erythropoietic islands in
the mesoderm of yolk sac. Those blasts {precursors} of the erythrocytes (and
some precursors of macrophage cells), however, are not multipotent and cannot
self-renew. The reason why those cells exist at that place and time is that the
embryonic developing tissues are in need of oxygen. After this stage is done,
we have the final hematopoiesis where erythroid myeloid progenitors (EMPs) are
produced. After them, we have the generation of Hematopoietic stem cells (HSCs)
which are multipotent and are produced in the area of Aorta Gonad Mesonephros
between the 10th and 12th day of the embryonic life. HSCs
will then migrate to the liver, the spleen, thymus and after birth they will
get “installed” in the bone marrow.
As mentioned above, HSCs are the precursor cells, are
multipotent that have less potential of renewal than their ancestors, but they
are the ones that give birth to all the different cells in our body. The
function and the development of those early stem cells are both influenced by the
microenvironment surrounding them. At first the HSCs cells are in the zero
phase (Go), existing in a quiescent state. In order for those cells to start
cell growth (G1) they need to get activated by small peptides, deposited point
for cell signal, cytokines. Especially, in the bone marrow, the cells require
more signals so cytokines start producing. In fact, there must be
a very specific environment consisted of macrophages*, fibroblasts, adipocytes
and hematopoietic growth factors such as erythropoietin, thrombopoietin, stem
cell factor and interleukins.
*Question: Macrophages are a type of white blood
cells and interleukins are made from different cell types that are produced in
more advanced stages of hematopoiesis. How is it possible for them to preexist
in the bone marrow before their ancestors even existing? If we assume that we
only have HSCs in our marrow, in order for them to produce blasts of each cell
type, their microenvironment must have macrophages and interleukins. How were
those cells produced since the differentiation has not developed fully yet?
Immediately after the appearance of cytokines and other important factors, this combination will lead the HSCs proliferation within certain pathways. In the end, they
will start generating blasts of different hematopoietic cell lines.
Erythrocytes
Everything begins
with a hematopoietic stem cell and erythropoietin that will help in the
differentiation towards the erythrocytes. Hematopoietic stem cells self-renew
and differentiate into a common myeloid progenitor and then to a megakaryocyte-
erythroid progenitor. Immediately after that, we have the Burst Forming Unit cells
(BFU-E) which will start differentiating very quickly and eventually will produce the Colony Forming Unit cells (CFU-E). CFU-E are in need of erythropoietin’s help
in order for them to proliferate and differentiate After some morphological changes,
proliferation, and divisions the first blasts on the erythroid line start
appearing. Those cells are called, proerythroblasts and will start
producing more and more cells following the row bellow.
Proerythroblasts à Basophilic erythroblast à Polychromatophilic erythroblast à Orthochromatic erythroblast à Reticulocyte à mature erythrocytes
Proerythroblast
Its cytoplasm gets
colored blue, has no granules and the nuclus occupies most of the cell’s space.
Eventually, it will produce two basophil erythroblasts.
There is a
general rule applied during cell differentiation. Every new cell line that is
produced, gradually shrinks is size, the nucleus becomes smaller while the
cytoplasm gets bigger.
Basophil Erythroblasts
The cytoplasm can get colored as blue, there are high
levels of RNA and the nucleus has gotten a bit smaller. The euchromatin is
visible and we can see it with a pinkish color. Lastly, Basophil Erythroblasts
can appear with an irregular shape and with pseudopods.
After the first mitosis two polychromatophilic erythroblasts(normoblasts) will be produced.
Polychromatophilic Normoblast
The cytoplasm has now gotten a greyish color, mainly because the RNA has decreased and there is also the addition of erythropoietin. Furthermore, the nucleus has been reduced a bit more in size. Also, here the appearance of heterochromatin is observed, with a blue color while the euchromatin holds a pink-red color. After two mitosis, four orthochromatic normoblasts will be produced.
https://era.library.ualberta.ca/items/676869c8-1256-4bfe-be4f-3bb82b8c9de6
Orthochromatic Normoblasts
In this cell type, there is an increased production of erythropoietin and the cytoplasm is starting to become less and less visible with a small number of polyribosomes. Also, the nucleus is small and dense and there are cytoplasmic folds. It is important to note that this cell does not go through division. It only matures.
https://era.library.ualberta.ca/items/7ceb86a8-4623-40a6-acbb-16e260c048a0
Reticulocyte
After the orthochromatic normoblast’s maturation
(expels the nucleus) we have the formation of reticulocytes. They do not have a
nucleus, but they consist of many ribosomes because it composes erythropoietin.
Gradually, the ribosomes and mitochondria will disappear, and erythropoietin’s production
will stop. Reticulocytes are bigger than
the cells that will be generated after them and there are more of them in the
bone marrow than in the blood (in a healthy organism). In fact, they remain in
the bone marrow for one to two days.
https://www.jaypeedigital.com/book/9789386107671/chapter/ch16
Erythrocytes
From erythrocytes, we
have the production of mature erythrocytes after the reticulocyte enters the
blood circulation.
https://www.youtube.com/watch?v=bbUlaTApuuI
Important stages of erythropoiesis
At some point, the erythroblasts shape a formation
called erythropoietic islands. Basically, erythroblasts proliferate and
differentiate inside of these «islands». These formations consist of one
central macrophage and his projections which surround and encircle the erythroblasts *. This structure has many roles such as nutritional role, providing support, supplying
the erythroblasts with iron to help with the production of erythropoiesis, they
are able to do phagocytosis of nuclei and abnormal normoblasts. In addition,
during erythropoiesis, the blasts express some adhesion molecules between
themselves and the macrophage, as well as some other protein molecules such as
EMP protein (erythroblast macrophage protein). Equally important is macrophage’s
production of cytokines and interleukins. All those molecules help with the
proliferation and the differentiation of the blasts. However, in cases of
chronic inflammation there is an increasement in the production of IL-6, TGF-b,
TNF-a. Their rise can cause an inhibition on the erythropoiesis.
The erythropoietin connects with
its receptor on the surface of the blasts during many times throughout the stages
erythrocyte’s maturation. Without it, the process could not be done correctly, or
the differentiation could have never taken place. Basically, after EPO binds
with its receptor, many signals will get activated and will lead to crucial for
the cell pathways. One pathway of utmost importance is the one that includes
STAT5, a transcription factor. This transcription factor will activate Jak2
kinase in order of it to start the proliferation of the blasts in the erythroid
cell line. In the same way, other kinds of transcriptions regulators such as Bcl-11a
control the transformation beta and gamma chain of embryonic hemoglobin. Therefore, the disturbance of those early
signal molecules (STAT5 etc) can lead to the appearance of MDS syndromes and
leukemias.
*A macrophage comes from a monocyte’s progenitor.
This indicates that in order for the erythrocytes to be finally made, a
macrophage should have been matured first. So, the hematopoiesis must have
started and be completed with the monocytes first. (?)
Sources
-My own notes
-Pediatric hematology -Oncology by Fotini Tzortzatou-Stathopoulou
-https://www.sciencedirect.com/topics/medicine-and-dentistry/hematopoiesis
-http://kremiotis.mysch.gr/BlastikaKyttara.pdf






0 comments